Essential Cleanroom Standards Every Pharma Facility Needs
Cleanrooms are essential facilities that provide controlled environments to minimize contamination in industries such as pharmaceuticals, biotechnology, medical devices, healthcare, and food production. These specially designed spaces regulate airborne particles, temperature, humidity, and microorganism levels, ensuring product quality and patient safety.
Cleanroom classification is crucial for establishing environmental management standards and meeting regulatory requirements set by organizations like ISO (International Organization for Standardization), GMP (Good Manufacturing Practice), FDA (U.S. Food and Drug Administration), EMA (European Medicines Agency), and WHO (World Health Organization).
Failure to adhere to cleanroom standards can lead to contamination, reduced efficacy, or even product recalls, severely affecting manufacturers and consumers. Therefore, understanding cleanroom classification is key in ensuring that pharmaceutical companies implement proper controls, maintain regulatory compliance, and uphold the highest quality standards in drug manufacturing.
Understanding Contamination Risks in Cleanrooms
Various factors, including personnel, equipment, raw materials, and the environment, can cause contamination in pharmaceutical cleanrooms. Contaminants are generally categorized into three main types:
- Particulate Contaminants – Dust, fibers, and airborne particles that could potentially compromise pharmaceutical formulations.
- Microbial Contaminants – Bacteria, fungi, and viruses that pose significant risks, especially in producing sterile formulations.
- Chemical Contaminants – Residues from cleaning agents, gases, or chemicals from the manufacturing process that may interact with pharmaceuticals.
These contaminants can compromise pharmaceuticals' safety and efficacy, making cleanroom classification extremely important in pharmaceutical manufacturing. By selecting the appropriate cleanroom classification, companies can meet industry standards and ensure the correct level of protection for their products.
Overview of Cleanroom Classification Systems
Cleanroom classification systems specify the acceptable levels of airborne particulate concentrations and microbial contamination in controlled environments. The two central classification systems used are:
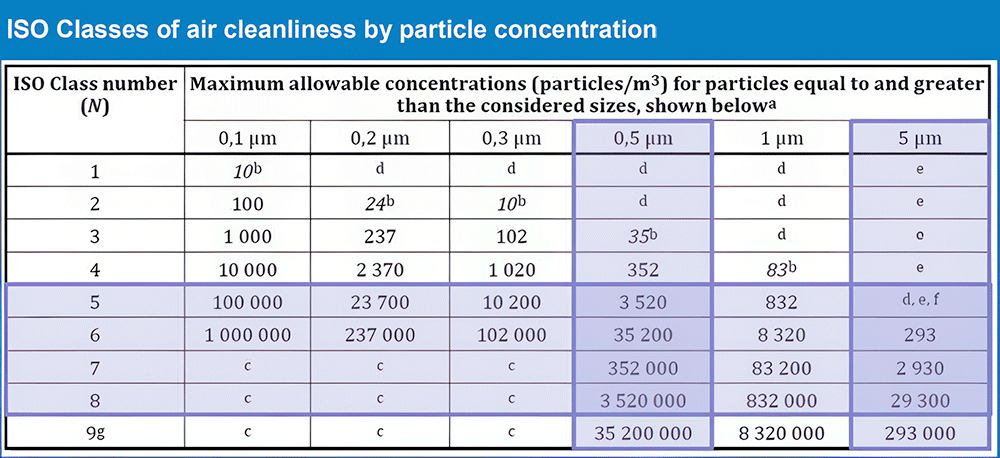
ISO 14644 Standards (ISO 1–9)
ISO 14644 is an international standard defining particle concentration levels in cleanrooms. Classifications range from ISO 1 (the cleanest) to ISO 9 (the most contaminated). In the pharmaceutical industry, ISO classes 5 through 8 are typically applied.
GMP Cleanroom Grades (A, B, C, D)
Good Manufacturing Practice (GMP) divides cleanrooms into four grades (A, B, C, D):
- Grade A – The most stringent requirements for areas requiring aseptic operations (e.g., sterile filling lines).
- Grade B – Cleanrooms used as background environments to Grade A areas.
- Grade C & D – Lower-priority areas that require certain cleanliness levels but not to the extent of Grade A or B.
Main Differences Between ISO Standards and GMP Classification
|
ISO Standard |
GMP Classification |
| Content of Standard |
Cleanroom classification based on the number of airborne particles |
Cleanroom classification that also considers the level of microbial contamination |
| Scope of Application |
Applicable to various industries, including pharmaceuticals |
Mainly applicable to the manufacture of sterile pharmaceuticals |
| Selection Criteria |
Applied according to product characteristics and regulatory requirements |
Applied according to the characteristics and regulatory requirements of the pharmaceuticals being manufactured |
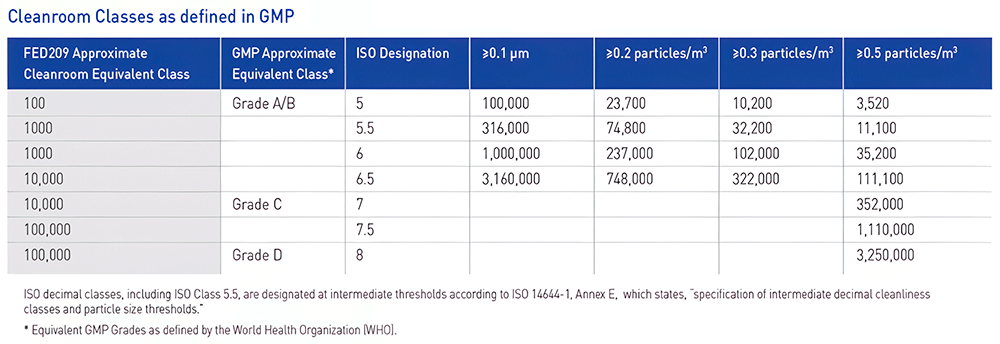
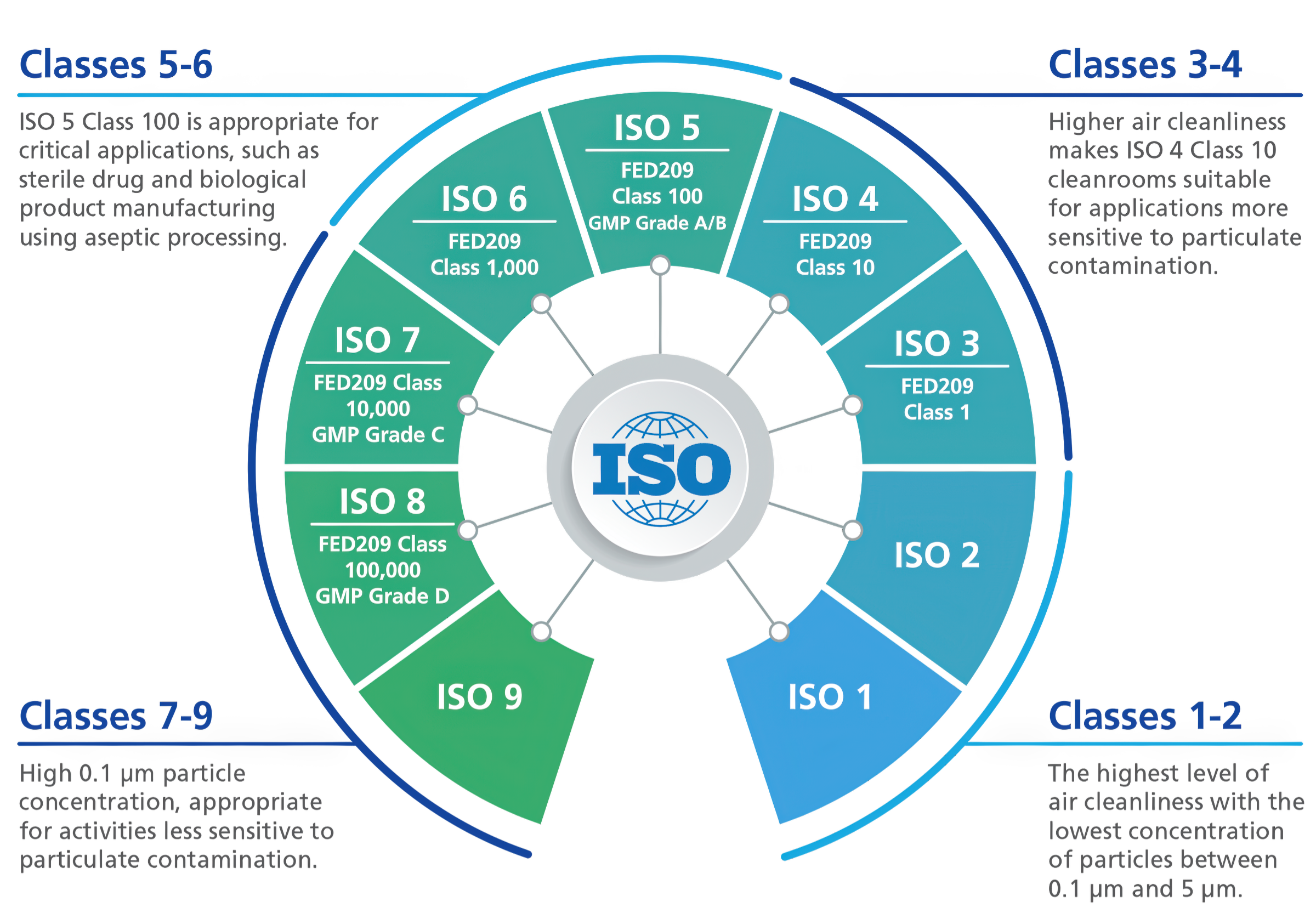
Both ISO 14644 and GMP (Good Manufacturing Practice) are essential standards in the environmental management of cleanrooms. ISO 14644 is an international standard that classifies cleanrooms from Class 1 to Class 9 based on airborne particle concentration and applies to various industries. On the other hand, GMP sets quality control standards specifically for manufacturing pharmaceuticals and biopharmaceuticals, and environmental control from GMP Grade A to D is required for aseptic manufacturing.
The figure below shows the correspondence between ISO classes and GMP grades. For example, ISO Class 5 corresponds to GMP Grade A/B, which is an environment with a high degree of cleanliness suitable for the manufacture of sterile pharmaceuticals. Thus, the pharmaceutical industry is required to properly manage the manufacturing environment while considering both ISO and GMP.
Cleanroom Classification Criteria in Detail
Pharmaceutical cleanrooms must meet multiple performance requirements to comply with regulations. Among these, particle count limitations are especially crucial.
Particle Count Requirements
Cleanroom classification is determined by the maximum allowable number of particles per cubic meter.
For example:
- ISO 5 Cleanroom: Allows up to 3,520 particles ≥0.5 µm per cubic meter.
- ISO 7 Cleanroom: Allows up to 352,000 particles of the same size per cubic meter.
As the ISO class number increases, the maximum allowable particle count rises, signifying a decrease in cleanliness. Proper particle management ensures the quality and sterility of pharmaceutical cleanrooms.
Airflow and Ventilation Requirements
Maintaining stringent airflow control is essential in high-grade cleanrooms. Laminar flow systems minimize turbulence by directing air uniformly in one direction, preventing contaminants from entering critical areas. In contrast, turbulent airflow systems diffuse air in multiple directions and are suitable for lower-grade cleanrooms where absolute sterility is not required.
Regular air changes are necessary to maintain cleanroom cleanliness. The frequency of air changes per hour (ACH) varies depending on the cleanroom classification, with higher-grade cleanrooms requiring more frequent air changes for effective particle removal.
Microbial Contamination Limits
GMP (Good Manufacturing Practice) classifications specify the maximum acceptable levels of microbial contamination within a cleanroom. For example, Grade A environments must not exceed 1 CFU (Colony Forming Unit) per cubic meter of air during operations.
Monitoring and Validation Protocols
Cleanroom performance is monitored using various testing methods. Airborne particle testing, conducted with laser particle counters, helps measure and control contamination levels. Microbial contamination is assessed through air and surface sampling, using settle plates and swabs to detect potential contamination risks. Additionally, HEPA and ULPA filter integrity testing ensures that filtration systems function correctly, maintaining a controlled environment.
Considerations for Pharmaceutical Cleanroom Design
Cleanroom design plays a pivotal role in contamination control. Walls, floors, and ceilings should have smooth, non-porous surfaces to prevent microbial growth and facilitate cleaning.
HVAC systems and air filtration units incorporate HEPA and ULPA filters, designed to capture airborne contaminants before they reach critical areas. Proper zoning and personnel management are also essential. Clearly designated entry and exit points, airlocks, and restricted movement patterns minimize contamination risks.
Additionally, pass-through chambers can be utilized to limit direct exposure during material transfer, further enhancing contamination control.
Cleanroom Classification: A Step-by-Step Guide
1. Risk Assessment and Planning
- Identify critical contamination risks.
- Determine the appropriate cleanroom classification based on regulatory requirements.
2. Construction and Qualification Phase
- Design Qualification (DQ): Confirm that the cleanroom design meets specifications.
- Installation Qualification (IQ): Verify that the cleanroom installation matches design requirements.
- Operational Qualification (OQ): Conduct testing for air filtration, particle count, and airflow performance.
- Performance Qualification (PQ): Verify ongoing compliance under actual operating conditions.
3. Continuous Monitoring and Compliance
- Perform regular particle testing and air sampling.
- Conduct regular disinfection and cleaning using validated methods.
- Provide employee training on gowning procedures and contamination control protocols.
Challenges and Mistakes in Cleanroom Classification
Cleanroom management can present various challenges. Misinterpreting ISO and GMP standards leads to compliance violations, posing risks for pharmaceutical manufacturers. Insufficient monitoring can cause deviations to go unnoticed, allowing contamination to spread undetected.
Human errors, such as improper gowning, can also introduce contaminants into the controlled environment. Additionally, inadequate equipment maintenance increases the risk of contamination-related malfunctions.
Proactively addressing these challenges is key to maintaining compliance and ensuring an efficient cleanroom environment.
Conclusion
Pharmaceutical cleanroom classification is essential for ensuring product safety, regulatory compliance, and contamination control. By implementing robust cleanroom standards, facilities can minimize risk while maintaining high-quality production.